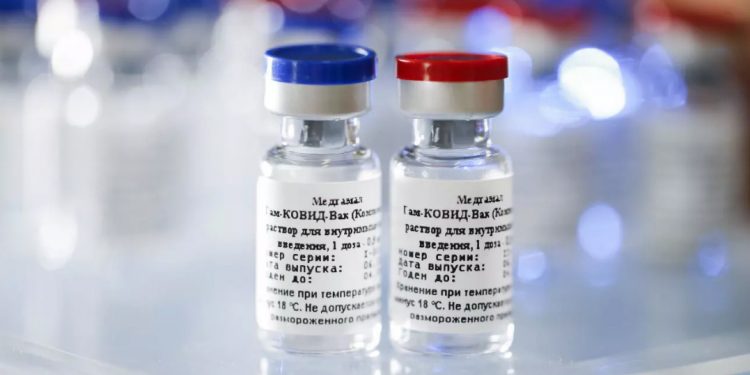
New Delhi: The Russian government has reached out to India seeking collaboration for manufacturing its COVID-19 vaccine ‘Sputnik V’. Russia also wants to conduct its phase 3 clinical trial of ‘Sputnik V’ in India, sources said Tuesday.
The matter was discussed by the national expert group on vaccine administration for COVID-19 in its meeting held August 22.
Sputnik V has been developed by the Gamaleya Research Institute of Epidemiology and Microbiology along with the Russian Direct Investment Fund (RDIF). The vaccine has not been tested in phase 3 or larger clinical trials. There has been scepticism in some quarters about limited data related to the efficacy of the vaccine.
“The Russian government has reached out to the Indian government seeking collaboration for manufacturing their COVID-19 vaccine, Sputnik V. It also wants to conduct its phase 3 trials here,” a source in the government said.
“The Department of Biotechnology (DBT) along with the Department of Health Research has been asked to lead and look into the matter. They (Russian government officials) have shared some information and data on Sputnik V. More data related to the safety and efficacy of the vaccine is awaited,” the source added.
Russian Ambassador to India Nikolay Kudashev has approached the office of Principal Scientific Advisor K VijayRaghavan regarding ‘Sputnik V’. This information was also given by sources who added that the envoy has approached secretaries of the departments of biotechnology and health research.
ICMR Director General Balram Bhargava talked about COVID-19 vaccine development in India at a press briefing Tuesday. He said currently two vaccine candidates, indigenously developed by Bharat Biotech in collaboration with ICMR and Zydus Cadila Ltd, have completed phase 1 of the human clinical trials and they will start phase 2 trials.