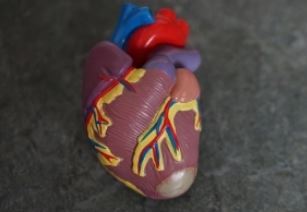
Toronto: Canadian researchers have grown a small-scale model of a human left heart ventricle – the main chamber of the heart – in the lab.
In the human heart, the left ventricle is the one that pumps freshly oxygenated blood into the aorta, and from there, into the rest of the body.
The bioartificial tissue construct is made with living heart cells and beats strongly enough to pump fluid inside a bioreactor, said the team from the University of Toronto.
The new lab-grown model, described in the journal Advanced Biology, could offer researchers a new way to study a wide range of heart diseases and conditions, as well as test potential therapies.
“With our model, we can measure ejection volume – how much fluid gets pushed out each time the ventricle contracts – as well as the pressure of that fluid,” said Sargol Okhovatian, a doctoral candidate in the varsity’s Institute of Biomedical Engineering.
“With these models, we can study not only cell function, but tissue function and organ function, all without the need for invasive surgery or animal experimentation. We can also use them to screen large libraries of drug candidate molecules for positive or negative effects,” added Milica Radisic, Professor in the department of chemical engineering and applied chemistry.
To grow the human cells in three dimensions (3D), the team used tiny scaffolds made from biocompatible polymers.
The model is a tube composed of three overlapping layers of heart cells that beat in unison, pumping fluid out of the hole at the end. The inner diameter of the tube is 0.5 millimetres and its height is about 1 millimetre, making it the size of the ventricle in a human foetus at about the 19th week of gestation.
Further, they measured the ejection volume and pressure using a conductance catheter, the same tool used to assess these parameters in living patients.
At the moment, the model can only produce a small fraction – less than five per cent – of the ejection pressure that a real heart could, but Okhovatian said that this is to be expected given the scale of the model.
“Our model has three layers, but a real heart would have 11,” she said.
“We can add more layers, but that makes it hard for oxygen to diffuse through, so the cells in the middle layers start to die. Real hearts have vasculature, or blood vessels, to solve this problem, so we need to find a way to replicate that.”
IANS