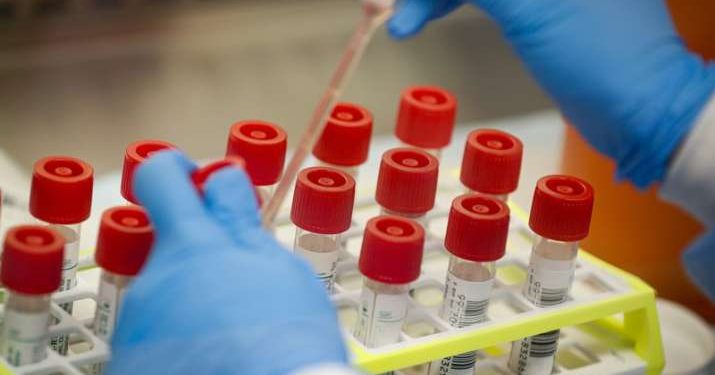
Seoul: South Korea’s Green Cross Corp (006280.KS) has received regulatory approval for phase II human clinical trials of its experimental coronavirus plasma treatment drug, the Ministry of Food and Drug Safety said Thursday.
The trials will test the safety and efficacy of the drug in 60 severe patients with underlying conditions like pneumonia, Green Cross said.
Green Cross was allowed to skip phase I trials. Its therapy is the country’s first to enter phase II for COVID-19 plasma treatment.
The overall number of global coronavirus cases has topped 22.3 million, while the deaths have increased to over 786,000, according to the Johns Hopkins University.
As of Thursday morning, the total number of cases stood at 22,322,208 and the fatalities rose to 786,185, the University’s Center for Systems Science and Engineering (CSSE) revealed in its latest update.
Agencies