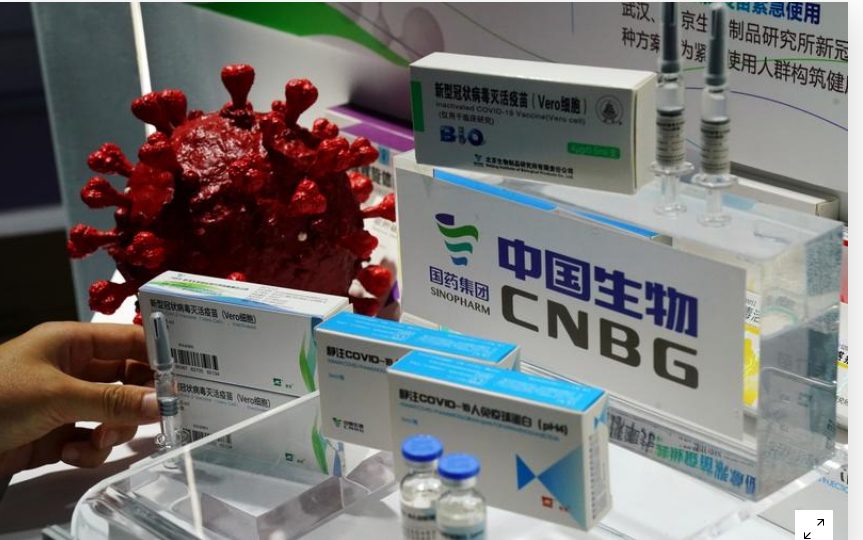
Beijing: China approved Thursday its first COVID-19 vaccine for general public use. The COVID-19 vaccine shot has been developed by an affiliate of state-backed pharmaceutical giant Sinopharm. Sources said that Beijing has given the approval as it braces for greater transmission risks over the winter.
No detailed efficacy data of the vaccine has been publicly released. But the vaccine developer, ‘Beijing Biological Products Institute’, a unit of Sinopharm subsidiary China National Biotec Group (CNBG), said Wednesday its vaccine was 79.34% effective in preventing people from developing the disease based on interim data.
Approval announced
The approval was announced by the National Medical Products Administration. It comes after the United Arab Emirates this month became the first country to roll out the vaccine to the public. Also Pakistan has announced a 1.2 million dose purchase deal with Sinopharm.
China has been slower than several other countries in approving COVID-19 vaccines. However, it has been inoculating some citizens for months with three different shots still undergoing late-stage trials.
Efficacy of Sinopharm shots
China launched an emergency use programme in July aimed at essential workers and others at high risk of infection. It administered more than 4.5 million doses as of December 15 using at least three different products. Two of the products have been developed by CNBG and one by Sinovac Biotech.
Also read: DCGI’s New Year wish raises hopes of COVID-19 vaccine approval soon
The efficacy of the Sinopharm shot trails the more than 90 per cent success rate of rival vaccines from Pfizer and Moderna. However, development of the COVID-19 vaccine points to progress China has made in the global race to develop successful COVID-19 vaccines.
Last stage trials
China has at least five vaccines in late stage trials. The vaccines have been developed by Sinovac, CNBG units, CanSino Biologics and the Chinese Academy of Sciences.
President Xi Jinping has pledged to make China’s vaccines a global public good. China has won several large supply deals with countries including Indonesia and Brazil.
Watching the data
The efficacy and safety data of China-made vaccines is being closely watched by many developing countries. They have limited early access to shots developed by Western drug makers. So they are looking for alternatives from China and Russia.
“China’s approval could boost the credibility of the vaccine,” said Dong-yan Jin, a professor at the University of Hong Kong. “But if the vaccine wants to take a share in the global market, especially in developed countries, more data is necessary,” Jin added.
A Sinopharm executive told a briefing detailed data would be released later. It would also be published in scientific journals at home and abroad.
The ‘South China Morning Post’ newspaper reported that China would vaccinate 50 million people from high-priority groups before the Lunar New Year holiday in February.
“We call on people … to take an active part in vaccination to protect themselves, family members and others,” Zeng Yixin, an official with National Health Commission, told a briefing Thursday. The price of the vaccine would depend on the scale of use, but the ‘premise’ was that it would be free for the public in China, he said.