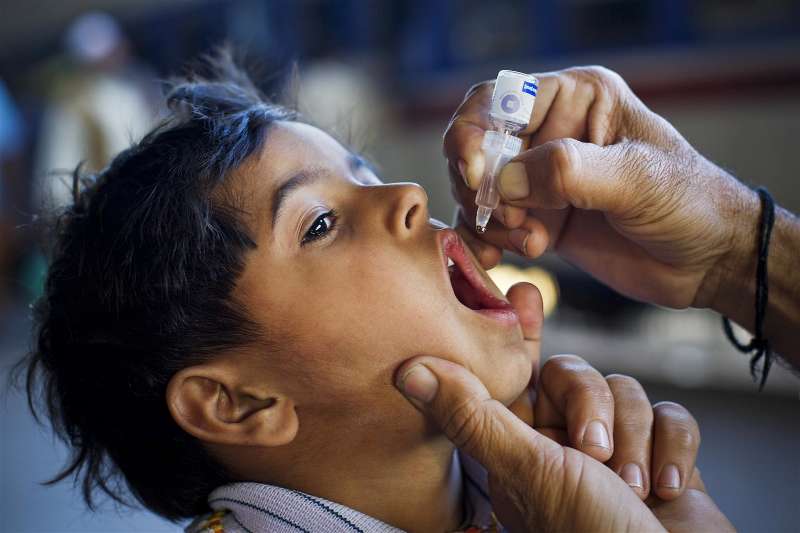
An oral, inactivated vaccine candidate against enterotoxigenic E. coli diarrohea has been found safe for infants and children in a clinical trial conducted in Bangladesh.
Enterotoxigenic E. coli (ETEC) bacteria are a primary cause of diarrohea, leading to substantial illness and death in children in low- and middle-income countries (LMICs) as well as in travellers to these countries.
Currently, there is no ETEC vaccine available on the market for use in either children or travellers to ETEC high-risk areas, and ETEC vaccine development is a World Health Organization priority.
The findings published in the journal The Lancet Infectious Diseases showed that all predefined primary endpoints for the study were achieved, showing that the vaccine candidate was safe and broadly immunogenic, stimulating immune responses to all key vaccine components.
The oral ETEC vaccine candidate, ETVAX, was developed at Sweden’s University of Gothenburg in collaboration with Scandinavian Biopharma, Stockholm.
ETVAX consists of inactivated E. coli bacteria expressing high levels of protective antigens and the ETEC-based B subunit protein LCTBA.
This clinical study examined the administration of ETVAX, given alone or together with different doses of an adjuvant, double-mutant heat-labile toxin (dmLT), to assess the vaccine’s safety and immunogenicity in 450 children.
The study involved infants and children from 6 months to 5 years of age.
In addition to safety analyses, immune responses were determined by measuring the amount of antibodies produced in the intestine (feces) as well as antibodies secreted by lymphocytes circulating in blood to the intestine.
The results confirm and extend the promising data previously reported from ETVAX trials in Swedish and Bangladeshi adults, said the study.