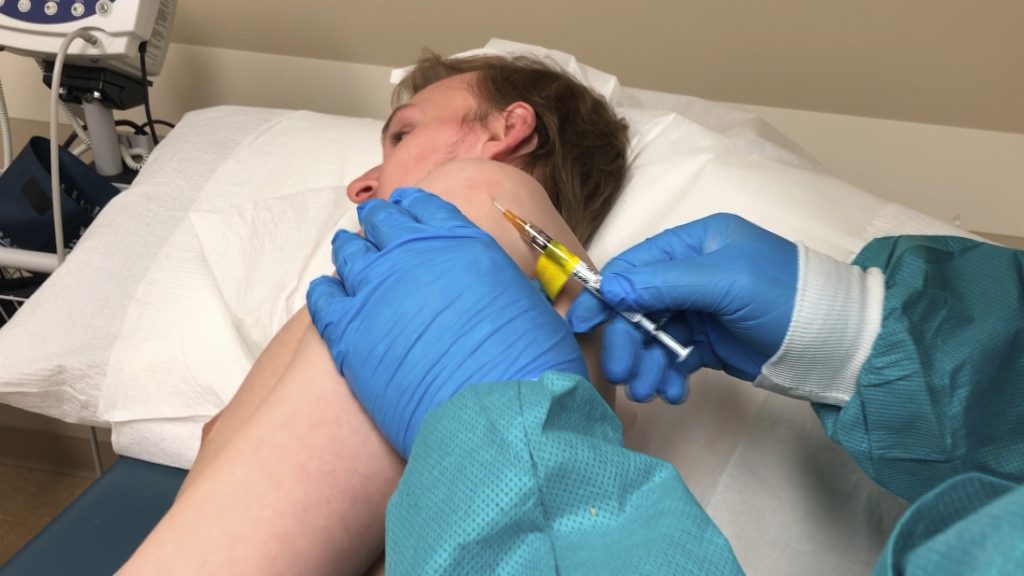
London: The World Health Organization (WHO) special envoy David Nabarro has warned that there is no guarantee that a coronavirus (COVID-19) vaccine can be successfully developed in coming months.
Nabarro who is one of the world’s leading experts said that people have to live with the threat of coronavirus “for the foreseeable future” and adapt accordingly because there is no guarantee that a vaccine can be successfully developed.
In an interview with The Observer, the WHO special envoy said the public should not assume that a vaccine would definitely be developed soon and would have to adapt to the ongoing threat.
“You don’t necessarily develop a vaccine that is safe and effective against every virus. Some viruses are very, very difficult when it comes to vaccine development – so for the foreseeable future, we are going to have to find ways to go about our lives with this virus as a constant threat,” he was quoted as saying.
“That means isolating those who show signs of the disease and also their contacts. Older people will have to be protected. In addition, hospital capacity for dealing with cases will have to be ensured. That is going to be the new normal for us all,” Nabarro added.
Nearly 44 COVID-19 vaccines are currently under various stages of development and scientists say that it will take 12-18 months before the world sees a successful one. According to the World Health Organisation (WHO), 42 vaccines are in pre-clinical stage while only two have entered Phase 1.
The two vaccines which are in Phase 1 are from CanSino Biological Inc. and Beijing Institute of Biotechnology; and Moderna/the National Institute of Allergy and Infectious Disease (NIAID) in the US.
Pharmaceutical giants Sanofi and GlaxoSmithKline (GSK) have also joined hands to speed up and develop an adjuvanted vaccine for COVID-19 disease.
The candidate vaccine is expected to enter clinical trials in the second half of 2020 and, if successful, would be available in the second half of 2021, the two companies said in a statement.
As of Sunday morning, the global number of coronavirus cases stood at 2,329,651, with 160,721 deaths.