Massachusetts(US): Researchers at the Massachusetts Institute of Technology (MIT) claimed to have developed a new self-administered injection “designed to provide long-term pregnancy prevention without the need for medical procedures or daily pills.”
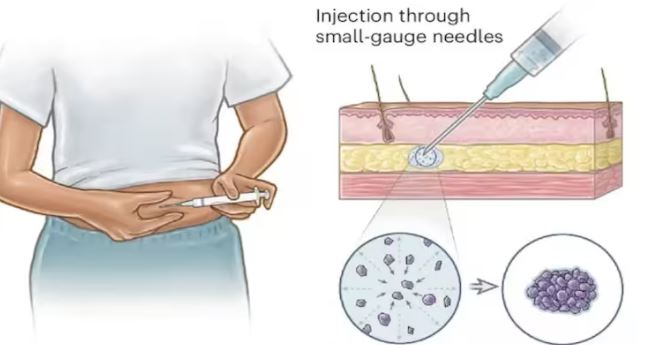
According to the researchers, the injection contains microscopic crystals that form under the skin and gradually release hormones to prevent ovulation.
Current contraceptive implants last for years but require surgical insertion by a trained professional, while contraceptive injections are only effective for about three months. This new approach aims to address both limitations. Although human trials have not yet begun, early results in animal studies are promising.
In tests conducted on rats, the drug was released for at least 97 days, with researchers suggesting the duration could be extended with further modifications.
“Specifically, in applications such as contraception, SLIM’s (self-aggregating long-acting injectable microcrystals) capability for prolonged drug release could dramatically reduce the frequency of administration compared with current self-administrable options,” stated the study published in Nature Chemical Engineering.
Dr. Giovanni Traverso, a co-author of the study, explained that a key challenge was ensuring the injection could be administered easily and comfortably at home.
“Our engineering challenge was finding a way to maximise comfort for patients by using smaller needles, which cause less bruising or bleeding,” Dr. Traverso said.
He also highlighted the potential benefits for individuals in low-resource settings, where daily pill consumption or access to contraception devices may be difficult.
“We anticipate that SLIM could be a new addition to the current suite of family planning options available to women, especially for people in low-resource settings where options for contraception and health care facilities are limited,” he added.
Beyond contraception, researchers believe this self-injectable technology could be used for other medical treatments requiring long-acting drug delivery, including HIV, tuberculosis, schizophrenia, chronic pain, and metabolic diseases.
“This is a very simple system in that it’s basically a solvent, the drug, and then you can add a little bit of bioresorbable polymer. Now we’re considering which indications do we go after: Is it contraception? Is it others? These are some of the things that we’re starting to look into as part of the next steps toward translation to humans,” Dr. Traverso stated.
Following these promising results, the researchers plan to advance their studies by conducting further preclinical trials to assess how the injection works in human skin.
PNN & Agencies