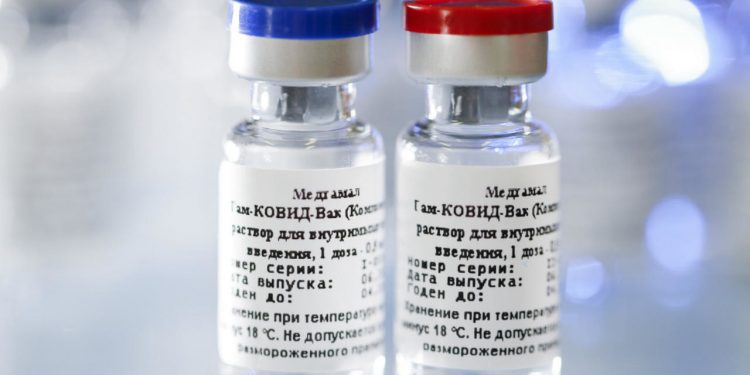
New Delhi: Scepticism has marked Russia’s approval of a COVID-19 vaccine. Several scientists around the world, including in India, suggest it hasn’t been tested properly. They also said that there may not be enough evidence to prove the efficacy of the COVID-19 vaccine.
Announcing the ‘breakthrough’ Tuesday, Russian President Vladimir Putin vouched for the safety and efficacy of the vaccine. It has been named ‘Sputnik V’. Putin said and said one of his daughters has already received a shot. Russia hopes to start mass-producing the vaccine by October. It plans to offer the first doses to essential workers. However, many in the science community seem unimpressed with Putin’s announcement.
The announcement should be taken ‘with a pinch of salt’, said Indian immunologist Vineeta Bal. “Some data must be out in the open for people to see. Among the data should be the clinical trial phases and numbers. If that is not done it is hard to believe that vaccine efficacy studies are successfully conducted between June 2020 and August 2020. I won’t at least,” Bal said. She is an immunologist from the Indian Institute of Science, Education and Research in Pune.
“Are they talking about controlled human challenge studies? If yes, that evidence is also useful to examine protective efficacy,” she noted.
Florian Krammer is a professor at the US-based Mount Sinai’s Icahn School of Medicine. He also questioned the safety of the vaccine.
“Not sure what Russia is up to, but I certainly would not take a vaccine that hasn’t been tested in phase 3. Nobody knows if it’s safe or if it works. They are putting HCWs (healthcare workers) and their population at risk,” Krammer said on Twitter.
Indian immunologist Satyajit Rath agreed with Krammer. Russian authorities seem to be depending on the generation of ‘neutralising’ antibodies in Phase 2 clinical trials for deciding that the vaccine candidate works, he said. A neutralising antibody defends a cell from an infectious particle by neutralising any effect it has biologically.
“While this is useful initial information, although even this is not really available in the public domain. So it is not evidence of protective efficacy. So effectively they are bringing vaccine candidates into use without actual evidence of efficacy,” Rath who works at the National Institute of Immunology here said.
Rath described the vaccine announcement as worrisome. He said it may not provide protection and may interfere with subsequent vaccine responses while causing problems for the recipients.
According to virologist Upasana Ray, the World Health Organisation (WHO) has asked vaccine developers to follow set guidelines.
“Efficacy is checked in Phase 2 but in a few hundred people or, in other words, in a small group. In Phase 3, this efficacy, safety etc, is checked in a larger group and monitored for infection possibilities. So, yes finishing Phase 2 is possible but not Phase 3,” Ray said. She is currently working as a senior scientist at Kolkata’s CSIR-Indian Institute of Chemical Biology (CSIR-IICB). noted.
Experts believe Phase 3 trials are where many promising vaccine candidates falter. A 2016 study, published in the journal ‘JAMA Internal Medicine’, analysed 640 Phase 3 drug trials in the US found that 344, or over 50 per cent, failed.
Ray said Russian authorities might have Phase 1 and 2 results, but completing Phase 3 completion so fast will be hard to believe unless the data is publicly available.
“They started their trials in June. August is yet not even halfway through. How did they finish Phase 3? Maybe they just checked the efficacy ie Phase 2 and now starting Phase 3 simultaneously with the release of the vaccine,” Ray pointed out.
According to the WHO, at least six other vaccine candidates – made by Sinovac, Sinopharm, Pfizer and BioNTech, the University of Oxford with AstraZeneca, and Moderna – have reached Phase 3 trials globally.
At least seven Indian pharma companies are also working to develop a vaccine against coronavirus.