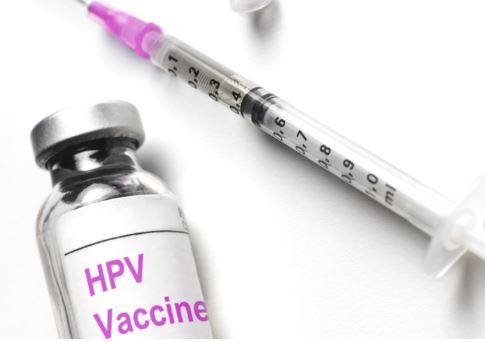
New Delhi: Serum Institute of India has informed the Centre that it can supply 1 crore doses of its indigenously developed Quadrivalent Human Papillomavirus vaccine (qHPV) against cervical cancer, by December 2022, official sources said Monday.
Prakash Kumar Singh, Director, Government and Regulatory Affairs, Serum Institute of India (SII) has written to the Union health ministry, confirming the supply of 1 crore doses of qHPV by December 2022 under the National Vaccination Programme, they said.
Singh has mentioned in his letter that SII will supply the vaccine at an affordable price, but cannot disclose the price before the online tendering process starts, they said.
The Drugs Controller General of India (DCGI) had July 12 granted market authorisation to SII to manufacture qHPV.
The phase 2/3 clinical trial of the vaccine has been completed with the support of the Department of Biotechnology.
The government advisory panel NTAGI recently learned to have also approved the qHPV after reviewing the clinical trial data of the vaccine.
In the application to DCGI, Singh stated that the qHPV vaccine CERVAVAC has demonstrated a robust antibody response that is nearly 1,000 times higher than the baseline against all targeted HPV types and in all dose and age groups.
The letter mentioned that lakhs of women are diagnosed every year with cervical cancer as well as a few other cancers, and the death ratio is also very high.
Cervical cancer in India ranks as the second most frequent cancer among women aged between 15 and 44 years.
“Also, it is noteworthy that presently, our country is fully dependent on foreign manufacturers for the HPV vaccine. In line with the philosophy of our group and under the leadership of our CEO, Dr Adar C Poonawalla, it has always been our endeavour to make available high-quality ‘Made in India’ vaccines at an affordable price for the people of our country and the world at large,” Singh had stated in the application.
PTI