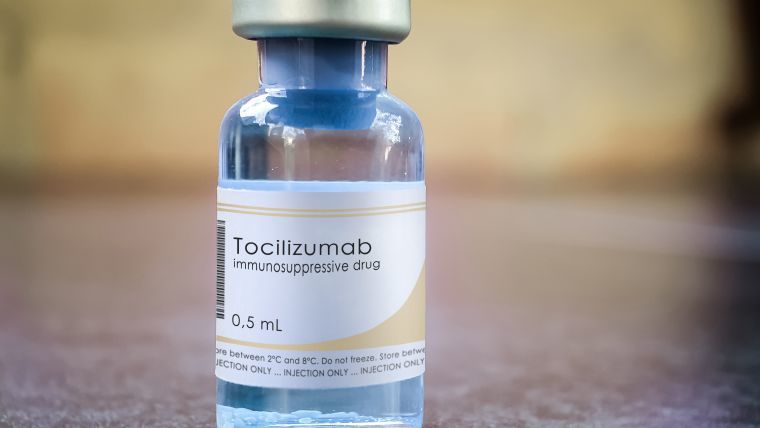
Bhubaneswar: The state health department Friday issued new guidelines for the rational use of Tocilizumab injection in treatment of COVID patients. Tocilizumab is a humanised antibody used as an immuno-suppressive drug.
This is often used in treatment of COVID patients in severe cases when the requirement of oxygen escalates and steroids also do not show any results.
The standard operating procedure (SOP) was issued by Additional Chief Secretary (Health) PK Mohapatra.
In his letter to all Collectors, he said, “As such the availability of the drugs are limited. So the treating doctors should take due diligence in prescribing new drugs / off-label drugs.”
Discussing the new SOP, he said, “It is observed that hospitals are submitting requisitions for provision of Tocilizumab for COVID-19 patients at different hours of the day without adequate information on the patient’s condition, investigation reports, indications, which is often making it difficult on the part of the Expert Committee to recommend its use and supply from the government source.”
“So as a measure to rationalise the supply of Tocilizumab for appropriate use by needy patients and to avoid its stockpiling, the hospital authority must submit their requisition for the patients along with the information in the prescribed pro forma addressed to MD, OSMCL,” Mohapatra said.
PNN